- 1Department of Gastrointestinal Surgery, Changhai Hospital, Naval Medical University, Shanghai, China
- 2Key Laboratory of Natural Medicines of the Changbai Mountain, Ministry of Education, Yanbian University, Yanji, China
As one of the most common forms of solid tumours, gastric carcinoma has been revealed as the third leading cause of death worldwide. The symptom of gastric cancer is usually not obvious and thus difficult to detect at earlier stages. Therefore, gastric cancer is already in the advanced stage once detected in patients, which has a poor prognosis due to ineffective therapies and multiple resistance. Recent advance in understanding the microenvironment of cancer has significantly promoted the development of immunotherapy for advanced gastric cancer. Immunotherapy can induce immune responses in gastric cancer patients thus leads to the destruction of cancer cells. In comparison of traditional therapy, immunotherapy has demonstrated robust efficacy and tolerable toxicity. Therefore, this novel strategy for treatment of advanced gastric cancer has gain increasingly popularity. In this review, we summarize recent progress of immunotherapy in advanced gastric cancer, such as immune check point inhibitors, adoptive cell therapy, VEGF inhibitors, cancer vaccines and CAR-T cell therapy. We highlight immunotherapies involved in clinical applications and discuss the existing challenges of current immunotherapies and promising strategies to overcome these limitations.
1. Introduction
Gastric cancer is the third leading cause of cancer-related death (1). Due to the delay in diagnosis and lack of effective therapies, patients with advanced gastric cancer suffer from poor prognosis and a short lifespan of approximately one year (2). The commonly used therapies of advanced gastric cancer are radiotherapy, chemotherapy and targeted therapy. Agents such as imatinib, larotrectinib, entrectinib and regorafenib are widely used for treatment of advanced gastric cancer (3, 4). However, multi-drug resistance and tumour relapse have largely limited the effectiveness of these traditional therapies.
In the recent years, immunotherapy has become a novel therapy to treat advanced gastric cancer and has quickly drawn the attention of researchers around the world owing to its amazing anti-tumour efficacy (5, 6). A better understanding of the tumour microenvironment has greatly facilitated the development of immunotherapies in advance gastric cancer (7). The most widely applied immunotherapies against advanced gastric cancer including immune checkpoint inhibitors (ICIs), adoptive cell therapy, cancer vaccine, vascular endothelial growth factor A (VEGFA) antibody and chimeric antigen receptor (CAR) T therapy, etc (8–10). Studies have shown that ICIs such as anti-PD-1/PD-L1 antibodies could effectively kill cancer cells via activation of the immune response (11). Clinical trials of ICIs have displayed efficacy and safety for cancer patients (12, 13). Notably, several ICIs such as pembrolizumab, avelumab, sintilimab, tislelizumab and ipilimumab have been approved for clinical application in combination with targeted therapy for treatment of advanced gastric tumour (14, 15).
In this review, we describe state-of-the-art development of immunotherapy for treatment of advanced gastric cancer, highlighting recent advances of ICIs, adoptive cell therapy, cancer vaccine and CAR-T cell therapy. In addition, we discuss the current challenges of immunotherapies, as well as potential strategies to overcome these limitations, such as combination of immunotherapy and targeted therapy.
2. Immunotherapy for Advanced Gastric Cancer
Over the past few years, a better understanding of the immune mechanism of gastric cancer has greatly facilitated the development of novel immunotherapies. ICIs could effectively interrupt the immune checkpoint interactions, leading to the destruction of tumour cells via activation of host’s immune system (16). The ongoing clinical trials of ICIs in advanced gastric cancer have been listed in Table 1. Other approaches such as adoptive cell therapy, VEGF inhibitors, cancer vaccines and CART cell therapy have also demonstrated potent anti-tumour activities (11, 17). These achievements in immunotherapy have marked a new era for advanced gastric cancer treatment (Figure 1).
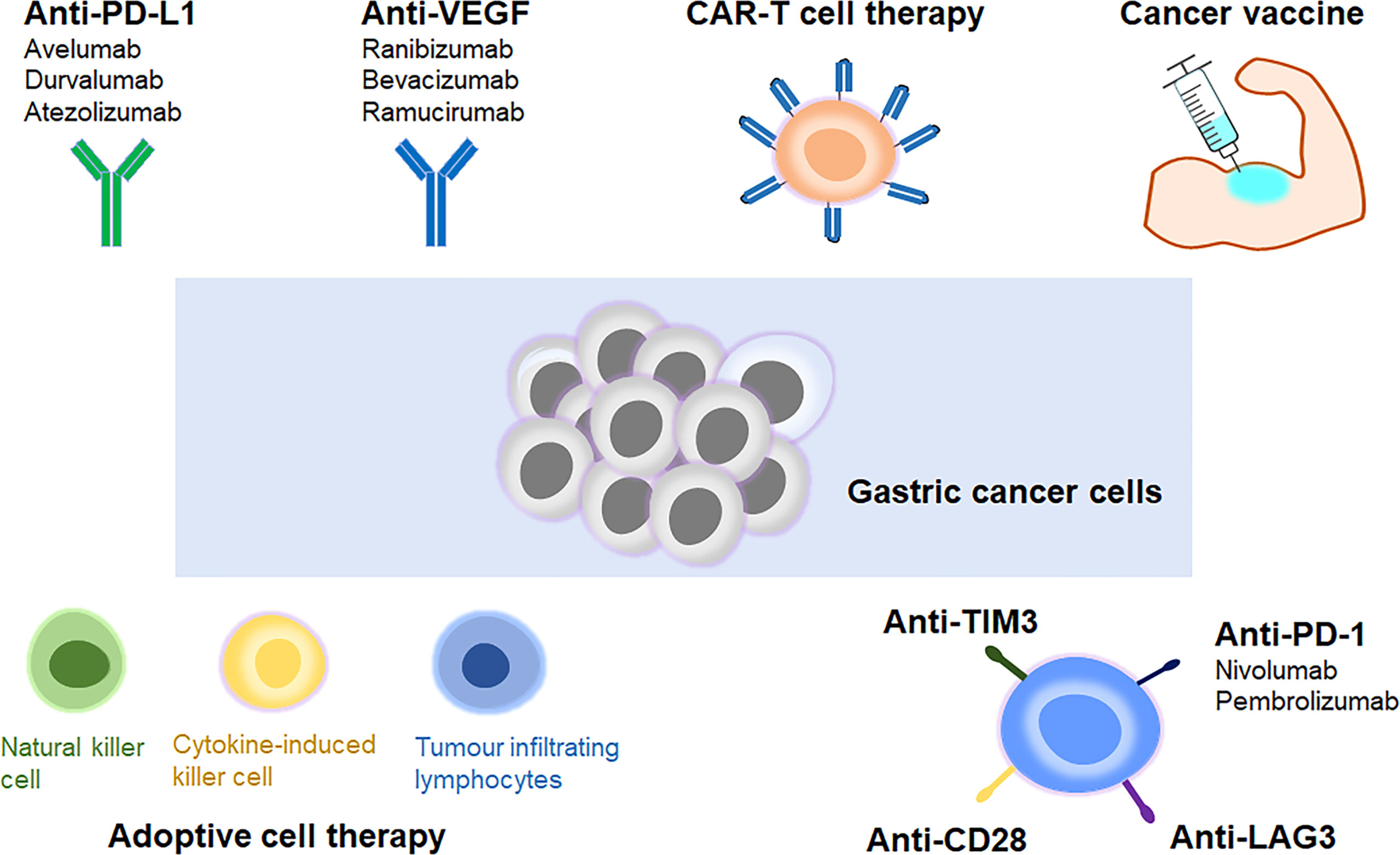
Figure 1 Different types of immunotherapies in advanced gastric cancer. Immune checkpoint inhibitor, adoptive cell therapy, VEGF inhibitor, cancer vaccine and CAR-T cell therapy are the main types of immunotherapies for treatment of advanced gastric cancer.
2.1 Immune Checkpoint Inhibitors
In 2011, ipilimumab became the world-first approved ICIs to treat melanoma (18). Since then, immune therapies have revolutionized the strategies for advanced gastric cancer treatment. There are mainly three types of ICIs, anti-PD1/PD-L1 and anti-CTLA4 antibodies (19). Activated immune cells such as T cells can express PD-1. PD-L1, the ligand of PD-1, binds to PD-1 thus resulted in immune cell apoptosis and immune suppression. PD-L1 is overexpressed in advanced gastric cancer, leading to the evasion of tumour cells from immune response (20). On the other hand, CTLA-4 protein can interact with B7-1/B7-2 with high affinity, leading to CD28 signalling pathway inhibition, which plays a critical role in T cell activation (21). Inhibitors targeting these immune checkpoints have been generated and studied in pre-clinical and clinical trials.
2.1.1 PD-1 Inhibitors
PD-1 inhibitor nivolumab is a monoclonal antibody that have gained the approval of FDA in the year 2014 for advanced gastric tumour treatment (22). The effects of nivolumab against advanced gastric cancer were examined via phase III clinical trials conducted over 40 countries in Asian (13). The initial results showed that nivolumab could significantly increase the survival rate of patients compared to the placebo. Nivolumab treatment in gastric tumour patients have demonstrated 12-month overall survival rates of 26.2% in contrast to that of 10.9% survival rates by placebo treatment, suggesting a promising cure for this poor prognosis population. Notably, nivolumab has been approved for clinical application as a novel approach to treat advanced and recurrent gastric cancer (22, 23).
Pembrolizumab is another promising inhibitor targeting PD-1. The efficacy of pembrolizumab has been assessed in the phase II trials among advanced gastric cancer (24). Treatment with pembrolizumab in advanced gastric cancer patients has demonstrated 11-month overall survival rates of 45.8%. In addition to its high anti-tumour activity, pembrolizumab has also shown moderate side effects. These advantages of pembrolizumab have prompted its approval for treatment of advanced gastric tumour in 2017 (25). Clinical trials of pembrolizumab in 592 patients with non-operable advanced gastric cancer have been conducted to determine the efficacy of pembrolizumab in comparison to paclitaxel (26). However, pembrolizumab alone didn’t demonstrate significantly improved survival rates compared to paclitaxel in patients. When pembrolizumab combined with paclitaxel, enhanced anti-tumour effect and better toleration was detected (27). Tislelizumab has also been assessed for its anti-tumour effect against advanced gastric cancer, providing hope for the evolution of PD-1-based immunotherapy in advanced gastric tumour (28).
2.1.2 PD-L1 Inhibitors
PD-L1 is overexpressed on various cancer cells and plays a crucial role in T cell inhibition (29). The well-known PD-L1 inhibitors include avelumab, durvalumab and atezolizumab (30). Avelumab is an anti-PD-L1 mAb which has demonstrated well toleration in the phase III trial among patients with advanced gastric cancer (31). Avelumab among patients from Japan have exhibited high overall response rates and survival rates. In addition, the efficacy of avelumab against advanced gastric cancer is enhanced in combination with other therapeutics. However, a phase I trial in advanced gastric cancer showed that atezolizumab was effective in one case out of 171 patients (10). The response rates in this clinical trial are closely related to PD-L1 expression. The mechanism of how PD-L1 inhibitors contribute to advanced gastric cancer may be that PD-L1 inhibition could activate DC cells, T lymphocytes and natural kill cells, thus leading to the destruction of gastric tumour (32).
2.1.3 CTLA-4 Inhibitors
CTLA-4 plays important roles in human immune system. CTLA-4 is homologous to CD28, but it can interact with B7-1/B7-2 with higher affinity (21). Therefore, CTLA-4 can regulate or even inhibit CD28 signalling. CTLA-4 inhibitors tremelimumab and ipilimumab have been evaluated in clinical trials of advanced gastric cancer (10). Evaluation of ipilimumab was performed in a phase II trial among advanced gastric cancer patients (33). However, this study was terminated because ipilimumab didn’t demonstrate significant improved survival rate compared with first line targeted agents. A clinical study of tremelimumab on 12 patients with non-operable advanced gastric cancer demonstrated moderate response rate, compared with a combined therapy using both tremelimumab and other anti-cancer agents. Of note, combined therapies targeting CTLA-4 and PD-1 have shown enhanced anti-tumour immunity (34). Combination therapy of ipilimumab and nivolumab has been approved to treat advanced gastric cancer. However, the efficacy of CTLA-4 inhibitor as a monotherapy in advanced gastric cancer remains to be further investigated.
2.2 Adoptive Cell Therapy
Gastric cancer cells can express specific neoantigens of high immunogenicity, thus leading to the activation of human immune system. In this way, cancer cells can be recognized and destroyed. However, cancer cells can generate suppressive factors including lymphocyte-activation gene 3 (LAG-3), TGF-β, prostaglandin E2 and IL-10 that inhibit immune response, thus escaping detection and clearance by the immune system (35). For patients whose immune systems fail to detect and response to cancer cells, adoptive cell therapy has been proved as effective strategies to treat advanced gastric cancer (36). Adoptive cell therapy utilizes various immune cells including tumour infiltrating lymphocytes (TILs), lymphokine-activated killer cells and cytokine-induced killer (CIK) cells to induce effective immunity to clear cancer cells (2, 37).
CIK cells are derived from peripheral blood lymphocytes in the presence of CD3 monoclonal antibodies, IL-2 and IFN-γ (38). The CIK cell population consists of CD3+CD56- T cell and CD3+CD56+ T cell, with high anti-tumour activity and proliferation activity (39). Moreover, CIK cells could generate cytokines and chemokines for the regulation and elevation of immune response. A preclinical study using CIK cells demonstrated strong anti-tumour activity of CIK (40). In addition, clinical trials of combined therapy using CIK cells and targeted therapy have shown increased effect against advanced gastric cancer (41).
TILs immunotherapy has been widely applied in advanced gastric cancer. In particular, TILs derived from gastric cancer in patients have been exposed to tumour specific antigens thus are extremely advantageous in immunotherapy. Clinical trials of adoptive cell therapy among gastric cancer patients have shown that combined therapies based on tumour-associated lymphocytes could increase the survival rate to 50% in comparation with using traditional therapy alone (42, 43). Furthermore, in recent years, expanded allogenic natural kill cells has also been used as a novel immunotherapy for treatment of advanced gastric cancer (44). Natural kill cells possess high anti-tumour activity and antibody-dependent cytotoxicity. However, the clinical application of natural kill cells in cancer treatment is severely limited by the lack of strategies to obtain a large amounts of functional natural kill cells (45). Further studies will be taken to investigate novel methods to generate sufficient natural kill cells for cancer immunotherapy.
2.3 Anti-Angiogenic Therapy
Vascular endothelial growth factor A (VEGFA) play essential roles in the development of gastric cancer via its involvement in the formation of new blood vessels, a process termed as angiogenesis (46). VEGFA functions in the modulation of cancer immune response, which could result in escape of tumour cells from the surveillance of the immune system (47). In addition, VEGF can promote the transfer of Treg cells to the sites of tumour. Clinical studies of combined therapies using VEGFA inhibitors and immune check point inhibitors among patients with advanced gastric cancer have shown promising effects, with enhanced anti-tumour effect and reduced toxicity. For instance, bevacizumab and ramucirumab can significantly prevent angiogenesis (48). Clinical studies of combined therapies using bevacizumab and ICIs such as atezolizumab, ramucirumab, durvalumab in advanced gastric cancer patients have shown favourable efficacy (48). These studies suggest that combined therapy using VEGFA inhibitors and ICIs targeting PD-1 or PD-L1 may shed light on the development of effective treatment in advanced gastric cancer.
2.4 Cancer Vaccines
Another novel immunotherapy in advanced gastric cancer is the application of cancer vaccines, which can activate immune responses against tumour cells in vivo (49, 50). Proteins and peptides are commonly used antigens to trigger immune responses. The most well-studied cancer vaccines are mRNA vaccines, which carries the genetic information of antigen and can translate it into protein rapidly to induce immune response, thus leading to the destruction of cancer cells (51). Studies have revealed that mRNA cancer vaccines showed strong efficacy and moderate side effects compared to traditional chemotherapy or targeted therapy (52). Moreover, combination of cancer vaccines and chemotherapies such as cisplatin and 5-fluorouracil have exhibited significantly enhanced cytotoxicity against tumour cells in preliminary clinical trials (53). A clinical study of HLA-A24 and HLA-A2 peptides examined the peripheral blood mononuclear cells in gastric cancer patients (54). Results showed that 50% of the patients treated with cancer vaccines had increased humoral and cellular response against vaccinated peptides.
2.5 CAR-T Cell Therapy
CAR-T cell is specifically designed for the expression of synthetic receptors that can induce T cells to detect specific cancer antigen, leading to the destruction of tumour cells via the host’s immunity (55). Biomarkers such as claudin 18.2 (CLDN 18.2), human epidermal growth factor receptor 2 (HER2), mucin 1, natural-killer receptor group 2 (NKG2D), epithelial cell adhesion molecule (EpCAM), mesothelin (MSLN) and carcinoembryonic antigen (CEA) play important roles in the diagnosis and function of gastric cancer (56). Studies have shown that CAR-T therapy can effectively target the above biomarkers for treatment of advanced gastric cancer (Table 2) (57).
HER2 is a surface antigen overexpressed in gastric cancer cells. HER2-postive gastric cancer usually exhibit multi-drug resistance that inhibit the anti-tumour activity of traditional agents. The development of drug resistance severely hampered the treatment of advanced gastric cancer (57). Of note, CAR-T therapy is an effective strategy to overcome the multiple resistance in advanced gastric cancer patients. Notably, studies of HER2 CAR-T therapy demonstrate high affinity for advanced gastric cancer. Clinical studies of CLDN18.2 CAR-T cells in CLDN18.2-positive patients-derived tumour models have demonstrated high anti-tumour activity (58). CA 72-4 is a surface glycoprotein highly expressed in advanced gastric cancer. CAR-T therapy targeting CA 72-4 has shown potent effect in tumour elimination (59). Therefore, CA 72-4 may be a potential target for advanced gastric cancer treatment. Notably, clinical studies in patients showed that CAR-T therapy in combination with other therapeutics displayed enhanced anti-tumour effects (57).
In addition, CAR-T cells targeting B7-H3 and CDH17 have made achievements in cancer treatment. Clinical studies have shown that B7-H3 is overexpressed in the tumour tissues of advanced gastric cancer patients and B7-H3 is strongly correlated to the advancement of gastric cancer. Anti-tumour effect of B7-H3 specific CAR-T cells has been evaluated in patients with advanced gastric cancer and demonstrated significant cytotoxicity against gastric tumour cells (60). CDH17 is a biomarker of gastrointestinal adenocarcinomas and plays key roles in CA2+-dependent adhesion switch and Wnt signalling. Recent progress in CAR-T cells targeting CDH17 has shed light on this novel immunotherapy as a potential safe and effective treatment for advanced gastric cancer. Pre-clinical studies using gastrointestinal carcinoma xenografts in mouse models have demonstrated that CDH17CART therapy has strong potency against advanced gastric cancer with no obvious toxicity to normal gastrointestinal epithelial cells (61).
3. Challenges and Potential Strategies
Development of immunotherapy in advanced gastric cancer has demonstrated great advantages over traditional therapies. However, there still exists various challenges that have severely limited the clinical application of immunotherapy in advanced gastric cancer, for instance, the side effects and toxicity of ICIs, cancer vaccines and CAR-T therapies.
ICIs can lead to autoimmune toxicities in cancer patients (62, 63). For example, the side effects of nivolumab including fatigue, pruritus and rash. Pembrolizumab treatment in advanced gastric cancer can lead to thyroid-related complications. In addition, ICIs can lead to high risk of transplant loss in patients with organ transplants. Although the side effects of PD-1/PD-L1 and CTLA-4 inhibitors are similar, PD-L1 inhibition can lead to more severe immune adverse events due to the loss of PD-L1 ability to bind to B7 (64). However, these side effects of immune checkpoint inhibition can be effectively prevented by immunosuppressive agents such as corticosteroids without impairing the clinical benefits of immunotherapy in advanced gastric cancer. Furthermore, combination of ICIs and targeted therapy display synergistic effects on advanced gastric cancer.
VEGF has been established as a crucial target for treatment of advanced gastric cancer. However, due to the wide expression of VEGF, side effects of VEGF inhibitors are commonly seen in clinic, including hypothyroidism, coagulation disorders, gastrointestinal perforations, hypertension, proteinuria, neurotoxicity (65).
Although cancer vaccine has shown favourable benefits in phase I and phase II trials against advanced gastric cancer, its clinical efficacy is low because of regulation and suppression from the host immune system. Novel strategies to overcome this limitation involve the development of combined therapies, for example, combination of cancer vaccine and immune modulator to avoid immune suppression, use of conventional chemotherapy in addition to cancer vaccine to enhance anti-tumour activity but reduce cytotoxicity (66).
Despite the amazing efficacy of CAR-T therapy against advanced gastric cancer, this novel treatment also exhibits strong toxicity that could be fatal (57, 67). The most commonly seen side effects of CAR-T therapy are known as cytokine release syndrome and CAR-T therapy-related encephalopathy syndrome that includes fevers, chills, nausea, headache, cardiac toxicity and neurotoxicity. Development of CAR-T cells with shorter lifespan or “on-switch” may effectively overcome the limitations of current CAR-T therapy, reduce the toxicity, and facilitate the wide clinical application of this novel immunotherapy in advanced gastric cancer (68).
4. Conclusion
Over the past decades, cancer immunotherapy has emerged as promising therapeutics for various cancers. Development of ICIs has been a breakthrough for advanced gastric cancer and demonstrated anti-tumour effect in patients. However, the toxicity and efficacy of immune checkpoint inhibition have largely limited its broad clinical application. Other immunotherapies including adoptive cell therapy, cancer vaccines and CAR-T cell therapy also showed anti-tumour activity in gastric cancer patients. Clinical trials of immunotherapy in combination with targeted therapy have shown enhanced anti-tumour activity and survival rate compared with using immunotherapy alone. Despite the advantages of immunotherapy in advanced gastric cancer, challenges such as moderate clinical efficacy and immune evasion blocks the broad application of immunotherapy in advanced gastric cancer. New strategies to overcome these challenges will involve combination of CAR-T therapy and ICIs, utilizing of immune modulators to avoid immune suppression. We believe that developing novel immunotherapy may shed lights on the treatment of advanced gastric cancer.
Author Contributions
DY, KY, and XC conceived the topic, revised and proofread the manuscript. XJ and ZL drafted the paper and prepared the figure and table. All authors approved the submitted version.
Funding
This study was supported by the Shanghai Science and Technology Funds (21Y11913100).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Pineros M, et al. Estimating the Global Cancer Incidence and Mortality in 2018: GLOBOCAN Sources and Methods. Int J Cancer (2019) 144(8):1941–53. doi: 10.1002/ijc.31937
2. Hogner A, Moehler M. Immunotherapy in Gastric Cancer. Curr Oncol (2022) 29(3):1559–74. doi: 10.3390/curroncol29030131
3. Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, et al. Five-Year Outcomes of a Randomized Phase III Trial Comparing Adjuvant Chemotherapy With S-1 Versus Surgery Alone in Stage II or III Gastric Cancer. J Clin Oncol (2011) 29(33):4387–93. doi: 10.1200/JCO.2011.36.5908
4. Ajani JA, Lee J, Sano T, Janjigian YY, Fan D, Song S. Gastric Adenocarcinoma. Nat Rev Dis Primers (2017) 3:17036. doi: 10.1038/nrdp.2017.36
5. Mellman I, Coukos G, Dranoff G. Cancer Immunotherapy Comes of Age. Nature (2011) 480(7378):480–9. doi: 10.1038/nature10673
6. Kono K. Advances in Cancer Immunotherapy for Gastroenterological Malignancy. Ann Gastroenterol Surg (2018) 2(4):244–5. doi: 10.1002/ags3.12184
7. Uppal A, Dehal A, Chang SC, Barrak D, Naeini Y, Jalas JR, et al. The Immune Microenvironment Impacts Survival in Western Patients With Gastric Adenocarcinoma. J Gastrointest Surg (2020) 24(1):28–38. doi: 10.1007/s11605-019-04403-w
8. Sharma P, Allison JP. Immune Checkpoint Targeting in Cancer Therapy: Toward Combination Strategies With Curative Potential. Cell (2015) 161(2):205–14. doi: 10.1016/j.cell.2015.03.030
9. Sharma P, Allison JP. The Future of Immune Checkpoint Therapy. Science (2015) 348(6230):56–61. doi: 10.1126/science.aaa8172
10. Xie J, Fu L, Jin L. Immunotherapy of Gastric Cancer: Past, Future Perspective and Challenges. Pathol Res Pract (2021) 218:153322. doi: 10.1016/j.prp.2020.153322
11. Kono K, Nakajima S, Mimura K. Current Status of Immune Checkpoint Inhibitors for Gastric Cancer. Gastric Cancer (2020) 23(4):565–78. doi: 10.1007/s10120-020-01090-4
12. Muro K, Chung HC, Shankaran V, Geva R, Catenacci D, Gupta S, et al. Pembrolizumab for Patients With PD-L1-Positive Advanced Gastric Cancer (KEYNOTE-012): A Multicentre, Open-Label, Phase 1b Trial. Lancet Oncol (2016) 17(6):717–26. doi: 10.1016/S1470-2045(16)00175-3
13. Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, et al. Nivolumab in Patients With Advanced Gastric or Gastro-Oesophageal Junction Cancer Refractory to, or Intolerant of, at Least Two Previous Chemotherapy Regimens (ONO-4538-12, ATTRACTION-2): A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet (2017) 390(10111):2461–71. doi: 10.1016/S0140-6736(17)31827-5
14. Janjigian YY, Kawazoe A, Yanez P, Li N, Lonardi S, Kolesnik O, et al. The KEYNOTE-811 Trial of Dual PD-1 and HER2 Blockade in HER2-Positive Gastric Cancer. Nature (2021) 600(7890):727–30. doi: 10.1038/s41586-021-04161-3
15. Takei S, Kawazoe A, Shitara K. The New Era of Immunotherapy in Gastric Cancer. Cancers (2022) 14(4):1054. doi: 10.3390/cancers14041054
16. Postow MA, Callahan MK, Wolchok JD. Immune Checkpoint Blockade in Cancer Therapy. J Clin Oncol (2015) 33(17):1974–82. doi: 10.1200/JCO.2014.59.4358
17. Coutzac C, Pernot S, Chaput N, Zaanan A. Immunotherapy in Advanced Gastric Cancer, is it the Future? Crit Rev Oncol Hematol (2019) 133:25–32. doi: 10.1016/j.critrevonc.2018.10.007
18. Robert C, Thomas L, Bondarenko I, O'Day S, Weber J, Garbe C, et al. Ipilimumab Plus Dacarbazine for Previously Untreated Metastatic Melanoma. N Engl J Med (2011) 364(26):2517–26. doi: 10.1056/NEJMoa1104621
19. Bagchi S, Yuan R, Engleman EG. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu Rev Pathol (2021) 16:223–49. doi: 10.1146/annurev-pathol-042020-042741
20. Jacob JA. Cancer Immunotherapy Researchers Focus on Refining Checkpoint Blockade Therapies. JAMA (2015) 314(20):2117–9. doi: 10.1001/jama.2015.10795
21. Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-Associated B7-H1 Promotes T-Cell Apoptosis: A Potential Mechanism of Immune Evasion. Nat Med (2002) 8(8):793–800. doi: 10.1038/nm730
22. Kato K, Satoh T, Muro K, Yoshikawa T, Tamura T, Hamamoto Y, et al. A Subanalysis of Japanese Patients in a Randomized, Double-Blind, Placebo-Controlled, Phase 3 Trial of Nivolumab for Patients With Advanced Gastric or Gastro-Esophageal Junction Cancer Refractory to, or Intolerant of, at Least Two Previous Chemotherapy Regimens (ONO-4538-12, ATTRACTION-2). Gastric Cancer (2019) 22(2):344–54. doi: 10.1016/S0140-6736(17)31827-5
23. Doi T, Iwasa S, Muro K, Satoh T, Hironaka S, Esaki T, et al. Phase 1 Trial of Avelumab (Anti-PD-L1) in Japanese Patients With Advanced Solid Tumors, Including Dose Expansion in Patients With Gastric or Gastroesophageal Junction Cancer: The JAVELIN Solid Tumor JPN Trial. Gastric Cancer (2019) 22(4):817–27. doi: 10.1007/s10120-018-0903-1
24. Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus-Acosta A, Delord JP, et al. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J Clin Oncol (2020) 38(1):1–10. doi: 10.1200/JCO.19.02105
25. Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, et al. Safety and Efficacy of Pembrolizumab Monotherapy in Patients With Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer: Phase 2 Clinical KEYNOTE-059 Trial. JAMA Oncol (2018) 4(5):e180013. doi: 10.1001/jamaoncol.2018.0013
26. Shitara K, Ozguroglu M, Bang YJ, Di Bartolomeo M, Mandala M, Ryu MH, et al. Pembrolizumab Versus Paclitaxel for Previously Treated, Advanced Gastric or Gastro-Oesophageal Junction Cancer (KEYNOTE-061): A Randomised, Open-Label, Controlled, Phase 3 Trial. Lancet (2018) 392(10142):123–33. doi: 10.1016/S0140-6736(18)31257-1
27. Shitara K, Van Cutsem E, Bang YJ, Fuchs C, Wyrwicz L, Lee KW, et al. Efficacy and Safety of Pembrolizumab or Pembrolizumab Plus Chemotherapy vs Chemotherapy Alone for Patients With First-Line, Advanced Gastric Cancer: The KEYNOTE-062 Phase 3 Randomized Clinical Trial. JAMA Oncol (2020) 6(10):1571–80. doi: 10.1001/jamaoncol.2020.3370
28. Zayac A, Almhanna K. Esophageal, Gastric Cancer and Immunotherapy: Small Steps in the Right Direction? Transl Gastroenterol Hepatol (2020) 5:9. doi: 10.21037/tgh.2019.09.05
29. Topalian SL, Drake CG, Pardoll DM. Immune Checkpoint Blockade: A Common Denominator Approach to Cancer Therapy. Cancer Cell (2015) 27(4):450–61. doi: 10.1016/j.ccell.2015.03.001
30. Doroshow DB, Bhalla S, Beasley MB, Sholl LM, Kerr KM, Gnjatic S, et al. PD-L1 as a Biomarker of Response to Immune-Checkpoint Inhibitors. Nat Rev Clin Oncol (2021) 18(6):345–62. doi: 10.1038/s41571-021-00473-5
31. Bang YJ, Ruiz EY, Van Cutsem E, Lee KW, Wyrwicz L, Schenker M, et al. Randomised Trial of Avelumab Versus Physician's Choice of Chemotherapy as Third-Line Treatment of Patients With Advanced Gastric or Gastro-Oesophageal Junction Cancer: Primary Analysis of JAVELIN Gastric 300. Ann Oncol (2018) 29(10):2052–60. doi: 10.1093/annonc/mdy264
32. Refolo MG, Lotesoriere C, Messa C, Caruso MG, D'Alessandro R. Integrated Immune Gene Expression Signature and Molecular Classification in Gastric Cancer: New Insights. J Leukoc Biol (2020) 108(2):633–46. doi: 10.1002/JLB.4MR0120-221R
33. Bang YJ, Cho JY, Kim YH, Kim JW, Di Bartolomeo M, Ajani JA, et al. Efficacy of Sequential Ipilimumab Monotherapy Versus Best Supportive Care for Unresectable Locally Advanced/Metastatic Gastric or Gastroesophageal Junction Cancer. Clin Cancer Res (2017) 23(19):5671–8. doi: 10.1158/1078-0432.CCR-17-0025
34. Kelly RJ, Lee J, Bang YJ, Almhanna K, Blum-Murphy M, Catenacci DVT, et al. Safety and Efficacy of Durvalumab and Tremelimumab Alone or in Combination in Patients With Advanced Gastric and Gastroesophageal Junction Adenocarcinoma. Clin Cancer Res (2020) 26(4):846–54. doi: 10.1158/1078-0432.CCR-19-2443
35. Gao X, Mi Y, Guo N, Xu H, Xu L, Gou X, et al. Cytokine-Induced Killer Cells As Pharmacological Tools for Cancer Immunotherapy. Front Immunol (2017) 8:774. doi: 10.3389/fimmu.2017.00774
36. Moreno V, Hernandez T, de Miguel M, Doger B, Calvo E. Adoptive Cell Therapy for Solid Tumors: Chimeric Antigen Receptor T Cells and Beyond. Curr Opin Pharmacol (2021) 59:70–84. doi: 10.1016/j.coph.2021.05.004
37. Jafferji MS, Yang JC. Adoptive T-Cell Therapy for Solid Malignancies. Surg Oncol Clin N Am (2019) 28(3):465–79. doi: 10.1016/j.soc.2019.02.012
38. Elia AR, Circosta P, Sangiolo D, Bonini C, Gammaitoni L, Mastaglio S, et al. Cytokine-Induced Killer Cells Engineered With Exogenous T-Cell Receptors Directed Against Melanoma Antigens: Enhanced Efficacy of Effector Cells Endowed With a Double Mechanism of Tumor Recognition. Hum Gene Ther (2015) 26(4):220–31. doi: 10.1089/hum.2014.112
39. Zanon C, Stocchero M, Albiero E, Castegnaro S, Chieregato K, Madeo D, et al. Multivariate Statistical Data Analysis as a Tool to Analyze Ex Vivo Expansion Dynamics of Cytokine-Induced Killer Cells. Cytometry B Clin Cytom (2014) 86(4):257–62. doi: 10.1002/cyto.b.21124
40. Zhang L, Zhao G, Hou Y, Zhang J, Hu J, Zhang K. The Experimental Study on the Treatment of Cytokine-Induced Killer Cells Combined With EGFR Monoclonal Antibody Against Gastric Cancer. Cancer Biother Radiopharm (2014) 29(3):99–107. doi: 10.1089/cbr.2012.1381
41. Jiang JT, Shen YP, Wu CP, Zhu YB, Wei WX, Chen LJ, et al. Increasing the Frequency of CIK Cells Adoptive Immunotherapy may Decrease Risk of Death in Gastric Cancer Patients. World J Gastroenterol (2010) 16(48):6155–62. doi: 10.3748/wjg.v16.i48.6155
42. Yoshida M, Ohtsu A, Boku N, Miyata Y, Shirao K, Shimada Y, et al. Long-Term Survival and Prognostic Factors in Patients With Metastatic Gastric Cancers Treated With Chemotherapy in the Japan Clinical Oncology Group (JCOG) Study. Jpn J Clin Oncol (2004) 34(11):654–9. doi: 10.1093/jjco/hyh120
43. Shen X, Zhou J, Hathcock KS, Robbins P, Powell DJ Jr., Rosenberg SA, et al. Persistence of Tumor Infiltrating Lymphocytes in Adoptive Immunotherapy Correlates With Telomere Length. J Immunother (2007) 30(1):123–9. doi: 10.1097/01.cji.0000211321.07654.b8
44. Cao B, Liu M, Huang J, Zhou J, Li J, Lian H, et al. Development of Mesothelin-Specific CAR NK-92 Cells for the Treatment of Gastric Cancer. Int J Biol Sci (2021) 17(14):3850–61. doi: 10.7150/ijbs.64630
45. Ishikawa T, Okayama T, Sakamoto N, Ideno M, Oka K, Enoki T, et al. Phase I Clinical Trial of Adoptive Transfer of Expanded Natural Killer Cells in Combination With IgG1 Antibody in Patients With Gastric or Colorectal Cancer. Int J Cancer (2018) 142(12):2599–609. doi: 10.1002/ijc.31285
46. Gabrilovich DI, Chen HL, Girgis KR, Cunningham HT, Meny GM, Nadaf S, et al. Production of Vascular Endothelial Growth Factor by Human Tumors Inhibits the Functional Maturation of Dendritic Cells. Nat Med (1996) 2(10):1096–103. doi: 10.1038/nm1096-1096
47. Liang L, Hui K, Hu C, Wen Y, Yang S, Zhu P, et al. Autophagy Inhibition Potentiates the Anti-Angiogenic Property of Multikinase Inhibitor Anlotinib Through JAK2/STAT3/VEGFA Signaling in non-Small Cell Lung Cancer Cells. J Exp Clin Cancer Res (2019) 38(1):71. doi: 10.1186/s13046-019-1093-3
48. Herbst RS, Arkenau HT, Santana-Davila R, Calvo E, Paz-Ares L, Cassier PA, et al. Ramucirumab Plus Pembrolizumab in Patients With Previously Treated Advanced non-Small-Cell Lung Cancer, Gastro-Oesophageal Cancer, or Urothelial Carcinomas (JVDF): A Multicohort, non-Randomised, Open-Label, Phase 1a/B Trial. Lancet Oncol (2019) 20(8):1109–23. doi: 10.1016/S1470-2045(19)30458-9
49. Wu H, Fu M, Liu J, Chong W, Fang Z, Du F, et al. The Role and Application of Small Extracellular Vesicles in Gastric Cancer. Mol Cancer (2021) 20(1):71. doi: 10.1186/s12943-021-01365-z
50. Kole C, Charalampakis N, Tsakatikas S, Kouris NI, Papaxoinis G, Karamouzis MV, et al. Immunotherapy for Gastric Cancer: A 2021 Update. Immunotherapy (2022) 14(1):41–64. doi: 10.2217/imt-2021-0103
51. Cafri G, Gartner JJ, Zaks T, Hopson K, Levin N, Paria BC, et al. mRNA Vaccine-Induced Neoantigen-Specific T Cell Immunity in Patients With Gastrointestinal Cancer. J Clin Invest (2020) 130(11):5976–88. doi: 10.1172/JCI134915
52. Pardi N, Hogan MJ, Weissman D. Recent Advances in mRNA Vaccine Technology. Curr Opin Immunol (2020) 65:14–20. doi: 10.1016/j.coi.2020.01.008
53. Ajani JA, Hecht JR, Ho L, Baker J, Oortgiesen M, Eduljee A, et al. An Open-Label, Multinational, Multicenter Study of G17DT Vaccination Combined With Cisplatin and 5-Fluorouracil in Patients With Untreated, Advanced Gastric or Gastroesophageal Cancer: The GC4 Study. Cancer (2006) 106(9):1908–16. doi: 10.1002/cncr.21814
54. Sato Y, Shomura H, Maeda Y, Mine T, Une Y, Akasaka Y, et al. Immunological Evaluation of Peptide Vaccination for Patients With Gastric Cancer Based on Pre-Existing Cellular Response to Peptide. Cancer Sci (2003) 94(9):802–8. doi: 10.1111/j.1349-7006.2003.tb01522.x
55. Lv J, Zhao R, Wu D, Zheng D, Wu Z, Shi J, et al. Mesothelin Is a Target of Chimeric Antigen Receptor T Cells for Treating Gastric Cancer. J Hematol Oncol (2019) 12(1):18. doi: 10.1186/s13045-019-0704-y
56. Caruso HG, Heimberger AB, Cooper LJN. Steering CAR T Cells to Distinguish Friend From Foe. Oncoimmunology (2019) 8(10):e1271857. doi: 10.1080/2162402X.2016.1271857
57. Bebnowska D, Grywalska E, Niedzwiedzka-Rystwej P, Sosnowska-Pasiarska B, Smok-Kalwat J, Pasiarski M, et al. CAR-T Cell Therapy-An Overview of Targets in Gastric Cancer. J Clin Med (2020) 9(6):1894. doi: 10.3390/jcm9061894
58. Song Y, Tong C, Wang Y, Gao Y, Dai H, Guo Y, et al. Effective and Persistent Antitumor Activity of HER2-Directed CAR-T Cells Against Gastric Cancer Cells In Vitro and Xenotransplanted Tumors In Vivo. Protein Cell (2018) 9(10):867–78. doi: 10.1007/s13238-017-0384-8
59. Zhang Q, Zhang Z, Peng M, Fu S, Xue Z, Zhang R. CAR-T Cell Therapy in Gastrointestinal Tumors and Hepatic Carcinoma: From Bench to Bedside. Oncoimmunology (2016) 5(12):e1251539. doi: 10.1080/2162402X.2016.1251539
60. Sun F, Yu X, Ju R, Wang Z, Wang Y. Antitumor Responses in Gastric Cancer by Targeting B7H3 via Chimeric Antigen Receptor T Cells. Cancer Cell Int (2022) 22(1):50. doi: 10.1186/s12935-022-02471-8
61. Feng Z, He X, Zhang X, Wu Y, Xing B, Knowles A, et al. Potent Suppression of Neuroendocrine Tumors and Gastrointestinal Cancers by CDH17CAR T Cells Without Toxicity to Normal Tissues. Nat Cancer (2022) 3(5):581–94. doi: 10.1038/s43018-022-00344-7
62. Bajwa R, Cheema A, Khan T, Amirpour A, Paul A, Chaughtai S, et al. Adverse Effects of Immune Checkpoint Inhibitors (Programmed Death-1 Inhibitors and Cytotoxic T-Lymphocyte-Associated Protein-4 Inhibitors): Results of a Retrospective Study. J Clin Med Res (2019) 11(4):225–36. doi: 10.14740/jocmr3750
63. Ramos-Casals M, Brahmer JR, Callahan MK, Flores-Chavez A, Keegan N, Khamashta MA, et al. Immune-Related Adverse Events of Checkpoint Inhibitors. Nat Rev Dis Primers (2020) 6(1):38. doi: 10.1038/s41572-020-0160-6
64. Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed Death-1 Ligand 1 Interacts Specifically With the B7-1 Costimulatory Molecule to Inhibit T Cell Responses. Immunity (2007) 27(1):111–22. doi: 10.1016/j.immuni.2007.05.016
65. Fukumura D, Kloepper J, Amoozgar Z, Duda DG, Jain RK. Enhancing Cancer Immunotherapy Using Antiangiogenics: Opportunities and Challenges. Nat Rev Clin Oncol (2018) 15(5):325–40. doi: 10.1038/nrclinonc.2018.29
66. Smith JP, Cao H, Chen W, Mahmood K, Phillips T, Sutton L, et al. Gastrin Vaccine Alone and in Combination With an Immune Checkpoint Antibody Inhibits Growth and Metastases of Gastric Cancer. Front Oncol (2021) 11:788875. doi: 10.3389/fonc.2021.788875
67. Bonifant CL, Jackson HJ, Brentjens RJ, Curran KJ. Toxicity and Management in CAR T-Cell Therapy. Mol Ther Oncolytics (2016) 3:16011. doi: 10.1038/mto.2016.11
Keywords: advanced gastric cancer, immunotherapy, immune checkpoint inhibitor, adoptive cell therapy, cancer vaccine, CAR-T cell therapy
Citation: Jin X, Liu Z, Yang D, Yin K and Chang X (2022) Recent Progress and Future Perspectives of Immunotherapy in Advanced Gastric Cancer. Front. Immunol. 13:948647. doi: 10.3389/fimmu.2022.948647
Received: 20 May 2022; Accepted: 06 June 2022;
Published: 01 July 2022.
Edited by:
Xuyao Zhang, Fudan University, ChinaReviewed by:
Shuai Wang, Harvard Medical School, United StatesLijie Zhao, University of Michigan, United States
Copyright © 2022 Jin, Liu, Yang, Yin and Chang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dongxiao Yang, dongxiaoyang2@gmail.com; Kai Yin, kyin67@smmu.edu.cn; Xusheng Chang, cxs20051014@163.com
 Xin Jin
Xin Jin Zhaorui Liu
Zhaorui Liu Dongxiao Yang
Dongxiao Yang Kai Yin
Kai Yin Xusheng Chang
Xusheng Chang